Quote of the Day
In order to write about life, first you must live it.
— Ernest Hemingway
Introduction
As my regular readers can tell, I do not passively sit and watch television. While I am watching a program (history or science-oriented, nothing else), I have my computer right there and I actively research what is being said during the program. Last weekend, I was watching an interesting program on the History channel called "How the Earth was Made". This particular program was about the formation of the Earth and it contained an excellent section on the generation of atmospheric oxygen (transcript of program). In my opinion, the star of the show was a little rocky structure called a stromatolite (Figure 1). A stromatolite is a layered, rock-like structure formed when shallow-water sediments are trapped in films of microorganisms.
Stromatolites can get quite large, such as this two-ton one reported here.
What piqued my mathematical interest was the following statement.
Over a period of 2 billion years, countless generations of stromatolites pumped out over 20 million billion tons of oxygen.
I was wondering if I could gain some additional insight into this number. In the main body of this post, I will look at how much oxygen is in our atmosphere today. In the appendix, I will examine how to estimate the total amount of oxygen over time that has been released on Earth. These will be rough order of magnitude calculations. Let's dig in ...
Analysis
Earth's Atmosphere and Oxygen
Initially, the Earth's atmosphere had very little oxygen (~25 million times less than today) – then came the "Great Oxidation Event", which started some 2.3 billion years ago. During this time, atmospheric oxygen levels rose dramatically. The oxygen was being pumped out by organisms like stromatolites. We know that this oxygen ended up in places other than the atmosphere. For example, I live in Minnesota where iron oxide is all over the northern half of the state – the amounts are staggering. There are also large amounts of oxygen dissolved in the ocean and embedded in the crust. This article discusses three mechanisms for incorporating atmospheric oxygen in the crust.
- iron reacting with oxygen-rich seawater and precipitating out.
- oxygen-rich water in seafloor sediments drawn into the crust.
- oxygen-rich sulfates in undersea hot springs reacted with iron in seafloor sediments.
How Much Oxygen is in the Atmosphere Today?
We live at the bottom of a sea of air. When we measure air pressure, we are really measuring the weight of the mass of air above that point. We can roughly calculate the mass of atmosphere by multiplying the air pressure times the area that pressure covers. We will break the Earth up into two parts: land and ocean.
We need to collect a few facts before we start our analysis.
- 20.95% oxygen by volume (Source - dry air value, I am ignoring water vapor)
- 70.8% of the Earth's surface is covered by water, 29.2% by land (Source)
- The mean radius of the Earth is 6,371.0 km (Source)
- The mean air pressure at sea level is 101.325 kP or 14.7 psi (Source)
- The mean elevation of the continents is 840 meters (Source).
- The mean air pressure at 840 meters is 91.633 kP or 13.3 psi (Source - equation used in Fig. 2)
With these facts in mind, it is now time to pull out the mathematical artillery (i.e. Mathcad).
Percentage of Oxygen By Mass
For ideal gases, the volume fraction and mole fractions are equal. The real atmosphere is not ideal, so let's go and compute the percentage of oxygen in the atmosphere by mass. To perform this calculation, we can use the gas composition of the atmosphere by volume and the molecular weights of these gases to compute the percentage of oxygen by mass. Equation 1 illustrates the calculation.
| Eq. 1 |
I used a table from the Wikipedia and did the Equation 1 calculation as illustrated in Figure 2.
Mass of Oxygen in the Atmosphere
Figure 3 shows the calculation for the total mass of oxygen in today's atmosphere.
Conclusion
Let's see if I can check my answers. According to the National Center for Atmospheric Research, the total mass of the Earth's atmosphere is 5.1353·1015 metric tons. So I have good agreement there. My estimate for the mass fraction of oxygen in the Earth's atmosphere (23.1%) equals that stated in the Wikipedia, so I am good there. My estimate for the total mass of O2 in the atmosphere is 1.2 million billion metric tons, which agrees with this reference. So overall the analysis is pretty close.
The stromatolites alone produced 20 million billion tons (possibly more, see Appendix). The majority of the oxygen produced by stromatolites has apparently gone somewhere other than the atmosphere. In fact, there are enormous amounts of atmospheric oxygen that ended up chemically tied up in rocks. Consider the following quote (Source).
While photosynthetic life reduced the carbon dioxide content of the atmosphere, it also started to produce oxygen. For a long time, the oxygen produced did not build up in the atmosphere, since it was taken up by rocks, as recorded in Banded Iron Formations (BIFs) and continental red beds. To this day, the majority of oxygen produced over time is locked up in the ancient "banded rock" and "red bed" formations. It was not until probably only 1 billion years ago that the reservoirs of oxidizable rock became saturated and the free oxygen stayed in the air.
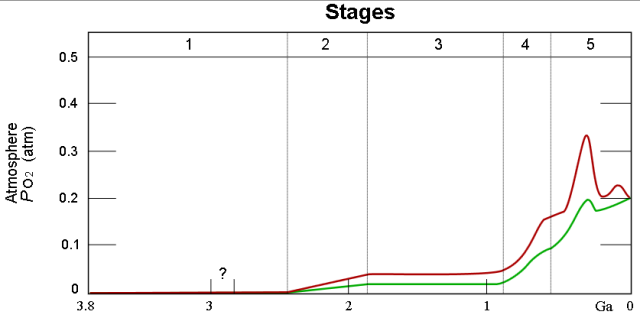
Figure 4 is from the Wikipedia and does a nice job illustrating the Earth's O2 level over time. The red and green lines illustrate the range of estimates for the percentage of O2.
There are some folks that claim the high oxygen levels in the past may have played a role in making dinosaurs so large.
Appendix A: Interesting Quote on Stromatolite-Produced Oxygen
Figure 5 shows where the photosynthesized oxygen went (Source). Note that this reference assumes that the total amount of photosynthesized mass of O2 on Earth is 3x1022 grams (=30 million billion metric tons). Clearly, estimates for the amount of photosynthesized O2 vary a bit.
The following quote (source) describes how most of the oxygen was chemically bound to the Earth and the rest was released to the air.
A sort of mass balance for atmospheric oxygen (including that dissolved in the sea) is shown in Figure 13.8 [my Figure 5]. The excess oxygen depends on a corresponding accumulation of organic matter (plus biologically reduced sulphur and methane) that has become buried in sedimentary deposits. About 58% and 38% of this oxygen has been used for the oxidation of iron and sulphur, respectively during the 3.5 - 3.8 billion years of organic photosynthesis. The remaining 4% (about 1.2x1015 tonnes) is found as free O2. The turnover rate of this atmospheric oxygen is only about 4000 years and is caused by organic photosynthesis and respiration processes that almost balance so that the biological turnover is vastly faster than the slow accumulation of organic matter. Given the crude estimates in Figure 13.5. there is altogether an excess of 3x1016 tonnes of oxygen (of which the greater part is in the form of oxidized iron and sulphur and only 4% as O2). Over roughly four billion years this corresponds to a net accumulation of 7.7x106 tonnes per year. The annual turnover due to photosynthesis and respiration corresponds to (1.2x1015[tonnes])/4000 [years] = 3x1010[11] tonnes O2 per year; that is production and respiration is about 20,000[36,500] times faster than the slow accumulation of organic matter in sedimentary rocks. This calculation, of course, assumes that both the production rate and the rate of accumulation of un-mineralized organic matter have been constant over geological time. Some evidence indicates that this has been the case since roughly the second half of the Precambrian.
As you can see from the quote, the calculation of total O2 production over the history of the Earth requires a number of assumptions.
I have noticed some minor math errors (see highlights) in the quote given above – minor in the sense that they do not change the result qualitatively, but I do see some calculation issues. Figure 6 shows my version of the calculations using the data given above. In the calculation, I assume that the period of organic photosynthesis was 3.65 billion years (the mean of the range given in the quote).






i love this!
If Stromatolites (Cyanobacteria) release Oxygen why are there 500+ dead Zones on earth and why do eutrophic lakes suffer from low Dissolved Oxygen.
Why are people complaining about algal (mainly cynaobacteria) blooms?
It's not because cyanobacteria release oxygen to the atmosphere while growing that they're a problem.
It's because when they die, they sink and their decomposition consumes oxygen from the water.
Eutrophication happens when an excess of nutrients is present, and cyanobacteria grow like crazy, outcompete everything, cover everything with slimy thick mats of algae ... and then die, sink, cover everything with slimy thick mats of dead algae, and rot, which last process consumes lots of oxygen. Also, they produce neurotoxins.
Here's a good description:
http://www.scienceclarified.com/El-Ex/Eutrophication.html
If today dead cyano decompose and draw down oxygen, then the same must have happened in the past. So how can you say that cyano contribute to the oxygen in the atmosphere?
If Stromatolites formed in the past, why are they not forming now?
Why are cyano now dying and decomposing instead of forming stromatolites?
Past is a indicator of present and vice versa.
Please think through the whole issue.
It's the growing like crazy part that's different. Cyanobacteria grow and die everywhere and mostly it's not a problem.
Eutrophication specifically is about excess nutrients, and in that situation the cyanobacteria really go nuts; they can respond to excess nutrient conditions much faster than higher plants and animals can. A lot of the problem dead zones are due to, basically, humans fertilizing the waters massively out of proportion to what the ecology in that area can normally take up, so the cyanobacteria fill in the gap.
If you read through the link I provided, you'll see that there are naturally eutrophic lakes as well. This is usually caused by weathering and erosion of high-nutrient rock and soil into a body of water. It's always happened; we're just doing it on a problematically large scale.
Realized on my way to work that I'd missed part of the question: "If Stromatolites formed in the past, why are they not forming now? Why are cyano now dying and decomposing instead of forming stromatolites?"
Stromatolites are fossil formations. First, only a very, very small fraction of things that die end up as fossils. Second, fossils form on a geologic time scale, not on a biologic time scale. It's entirely possible that some of today's dying cyanobacteria will eventually end up as fossils in a billion years or so.
Also, when stromatolites formed, there were no grazers––fish, snails, and other creatures that eat cyanobacteria––around. Today, grazers eat cyanobacteria and, except where waters are hypersaline (Shark Bay, Australia for example), stromatolites can't form.
You remind me of.......me, i viewed And enjoyed that same program. Additionally your Calcutions And Math. I used them against some of my own calculations were in I used an old garage measuring 25x20x8'tall. After insulating it i monitored The evening tempetures within for three days at specfic times with regular inside tempetures being obtained. On day four i brought in my 2010 Ford Hot from driving, turning it off,parked And closed it up to see just how much heat was being released and the effect rise in temp. On the square footage. Unbelievable the amount of heat from, transmissions, catyalatic converters, engine blocks, tail pipes, radiator etc. raised the temp. I didn't even have it running. I once saw a giant ballon filled with emissions from exhaust from a car scientists where collecting to estimate the billions of pounds of fossil fuel emissions and soon after they developed converters a platinum dirivitive palladium. Cars are heating the earth and will create global cooling ultimately like freezing hot water opposed to cool water. The transformation the electric cars can not happen fast enough. These opinions are my own.
I would like to thank you for the efforts you have put in writing this website.
I really hope to view the same high-grade content by you later on as well.
In fact, your creative writing abilities has motivated me to
get my own, personal blog now 😉
When you start your blog, send me a link. I would love to read what you write.
mark
What is the total mass of dissolved oxygen in water on Earth?
Oxygen levels in the ocean are going down. Is this significant compared to atmospheric oxygen?
Cyanobacteria feed on carbon dioxyde (CO2) and they were responsible for the conversion of the original thick atmosphere with 95%+ CO2 (same as on Mars and Venus) into oxygen. Without them no oxygen would not have existed on Earth and all of the water would have been decomposed and hydrogen lost to space. Testimony of these past events on a geological scale are the dolomite formations (anhydrous carbonate mineral composed of calcium magnesium carbonate) that have captured massive amount of CO2 originally in the atmosphere. This is actually not a good period for the cyanobacteria since the CO2 content of the atmosphere is at an all time low. Under thick atmospheric conditions the CO2 was one million time more abundant in the atmosphere than it is today.
https://is.gd/wPxb20
Great link!
Thanks.
mark
Inspiration...
There is Presently Only Six Times More Carbon in the Earth Atmosphere than in all the Terrestrial Plants Biomass
Thanks for the article. Some questions come to my mind. What is the relative magnitude of brush fires (as in California, Brazil, etc.)? What about one nuclear blast? What about a nuclear war? If the world-wide fires from a nuclear exchange reduces oxygen on a similar magnitude could the change in the oxygen level be reduced to a level below what we need to survive? It's chilling to ponder the possibility that you don't have to live in a country that was not bombed because the problem could reach you anywhere.