Quote of the Day
I like opera, I just don't want to be around the people who like opera.
— Justice Clarence Thomas, during a discussion of Justice Scalia and Scalia's love of opera. I also have experienced being around engineers who love opera – Justice Thomas is correct.
Introduction
During my editing of a previous post on heart power, which assumed that the heart converts chemical to mechanical energy with an efficiency of 20%, I found some other information on the web that would allow me to estimate the heart's conversion efficiency. I always like to do calculations that cross-check one-another to ensure that my information is consistent. This post documents my heart efficiency estimation exercise.
I was impressed with the wide variation that exists in the heart's physical parameters between individuals. This analysis is going to be rough because of the wide variability in these heart parameters.
Analysis
Approach
The analysis approach is basic.
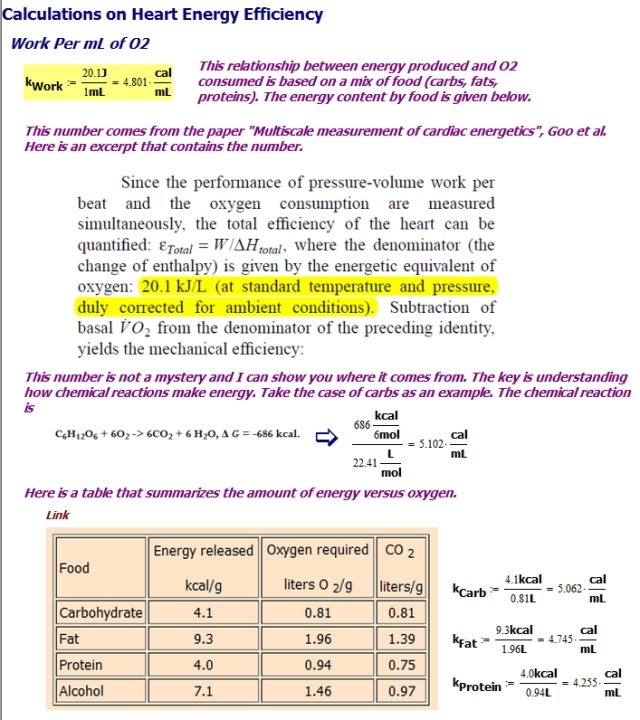
- Using information on the chemistry of food, determine the amount of energy produced by carbohydrates, proteins, and fats for every mL of O2.
This information is well documented from numerous source (example). The amount of energy available per liter of O2 varies with the type of carbohydrate (e.g. glucose, fructose), fat (e.g. stearate, palmitate), or protein (e.g. alanine, aspartate).
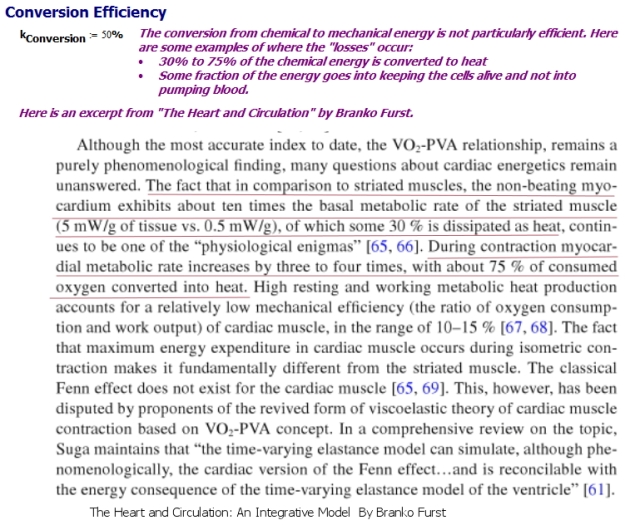
- Determine how much of the food energy is actually available for pumping versus heat generation and keeping cells alive.
This is measured value and it will vary widely based on the individual and the heart's activity level.
- Obtain the oxygen input to the heart.
This value has to measured under laboratory conditions.
- Compute the chemical power consumed by the heart and compare it to the mechanical work generated by the heart.
Given a specific heart size, food mix, and O2 consumption rate, we can determine the rate of chemical energy production. Since I computed the mechanical pumping power here, we can compute the efficiency.
Heart's Work Per mL of Oxygen
Figure 2 shows how to obtain an estimate for the average work performed by the heart per mL of O2. Notice how the number varies with the composition of the nutrients (carbohydrates, fats, proteins) that are feeding the heart.
Heat and Cell Overhead Losses
Figure 3 shows the numbers I found for the energy losses the heart experiences because of heat generation and cell overhead (i.e. keeping heart cells alive).
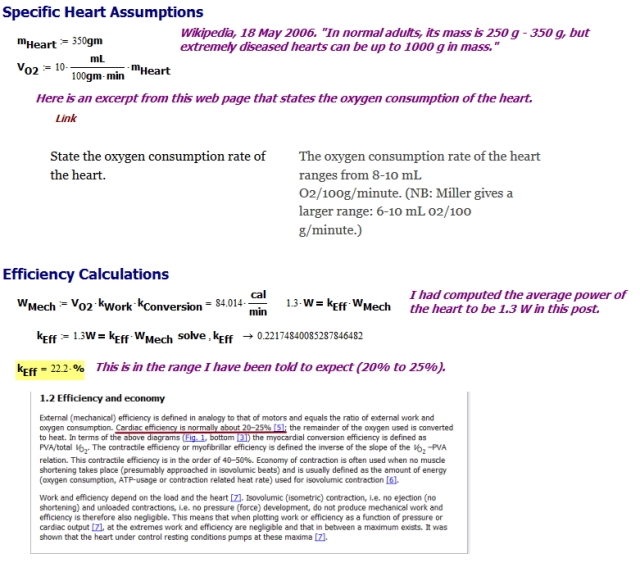
Chemical-to-Mechanical Efficiency Calculation
In this post, I estimated the heart's mechanical output power at 1.3 W. We can use this number along with the average heart size and oxygen consumption to estimate the chemical-to-mechanical conversion efficiency (Figure 4).
I compute an efficiency of 22%, a number that is highly dependent on a number of assumptions.
Conclusion
My plan for this post was to use some basic physics and a few pieces of information from the web to estimate the heart's chemical-to-mechanical conversion efficiency. My estimate of 22% is in within 20% to 25% range given by numerous sources. This demonstrates that the information I have been reading is internally consistent.


Pingback: Heart Energy Per Day Versus Truck Energy Consumed Over 20 Miles | Math Encounters Blog
Approximately 60% of ATP hydrolysis fuels directly heart pump (contractile shortening), and the remaining 40% is primarily used for the ion pumps (DOI: 10.1152/physrev.00006.2004). Considering 1.3 W power, mean mass of 300 g, metabolic rate 400 kcal/kg/day , heart's chemical-to-mechanical conversion efficiency is higher, up to 37%. However, conversion of food energy to ATP is not 100%, I suggest 66% based on free energy ATP hydrolysis under physiological conditions of -70 kj/mol, starting from glucose as food. Moreover, heart prefers fatty acids as source of energy (60–90% of the acetylCoA comes from beta-oxidation of fatty acids), so the math is more complicated if we calculate the chemical efficiency starting from food energy. Just a biochemist point of view!
Very cool. I just finished reading a book about theories on how life on Earth began. Much of the book was about theories on how the ATP/ADP cycle started. I hope to do more reading on this subject. I have developed a great respect for the complexity of these life cycles.
mark